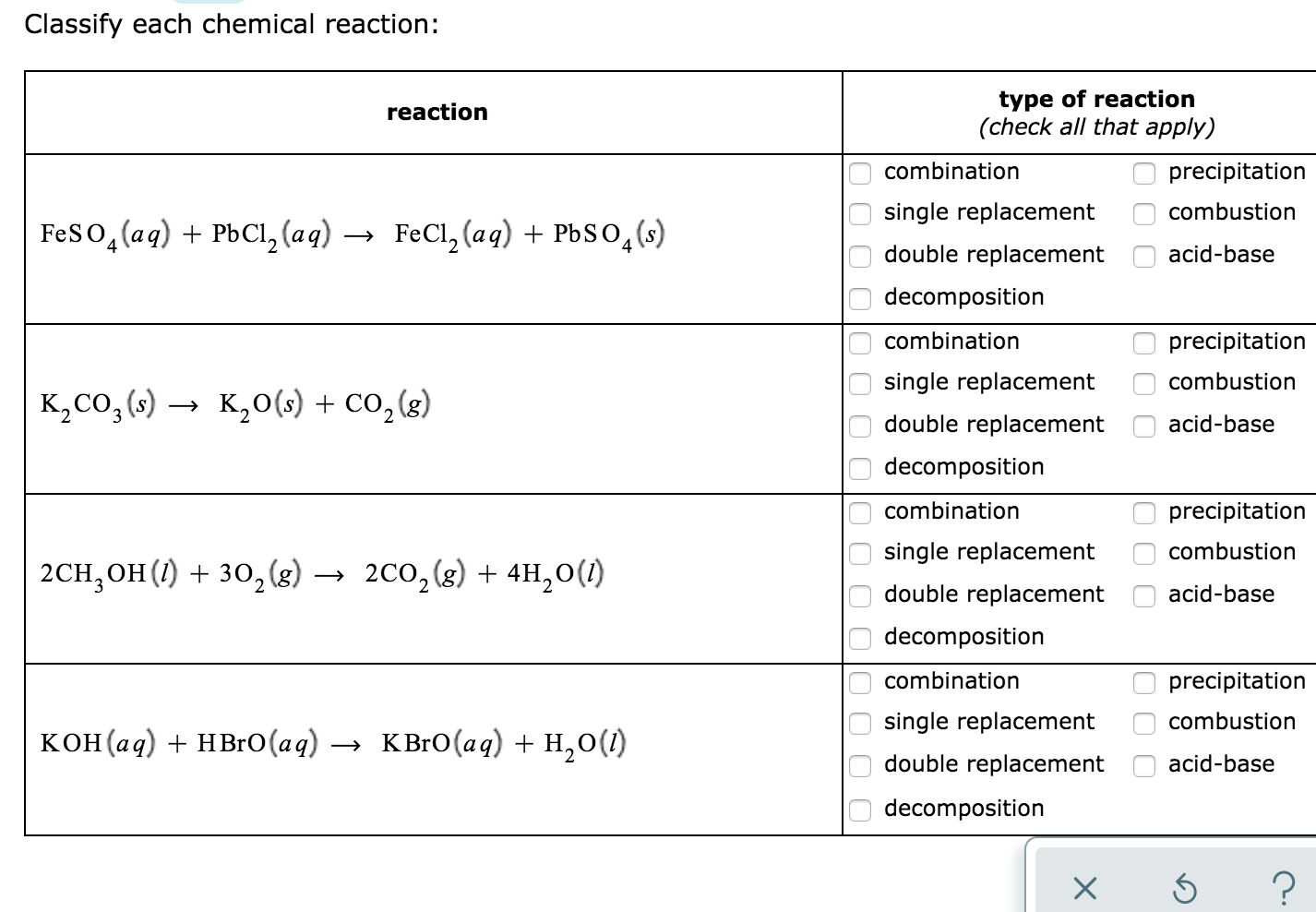
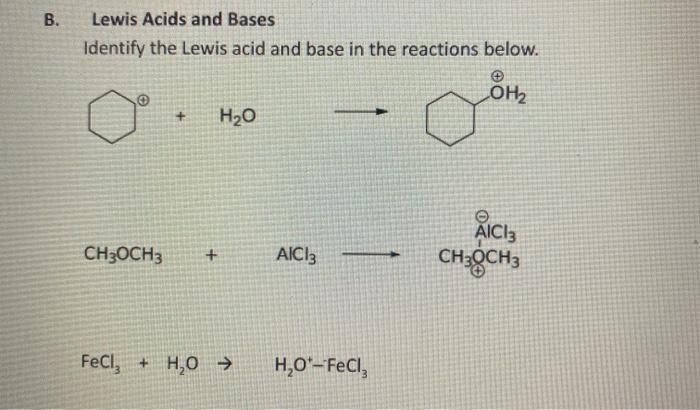
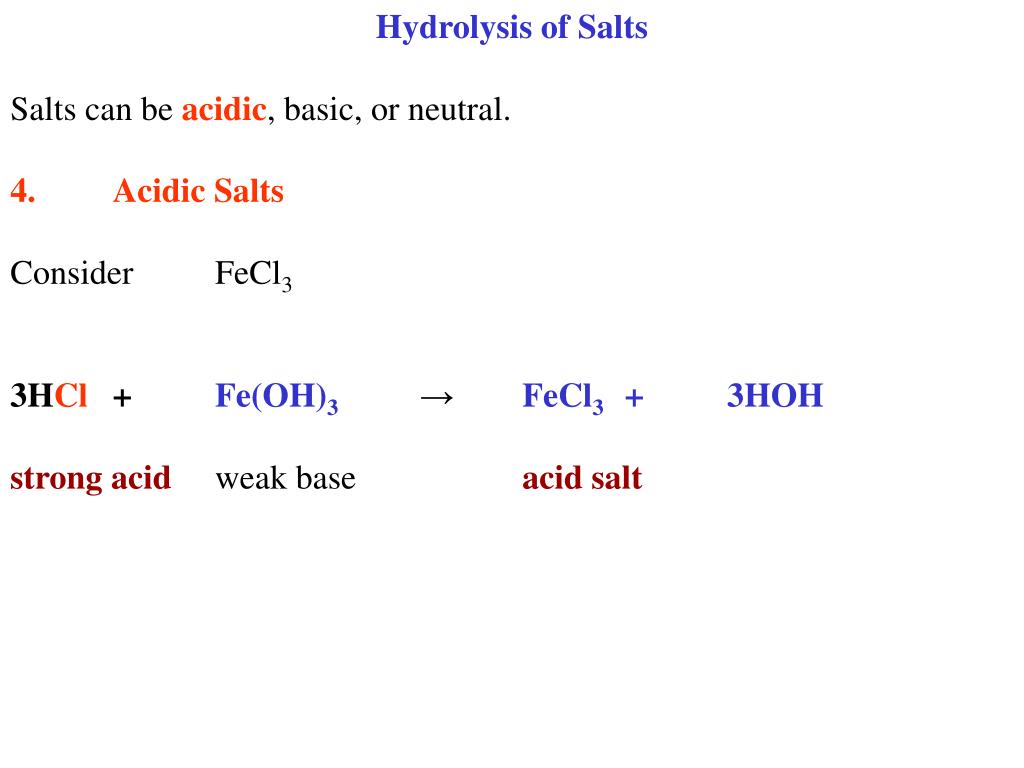
OneClass: Classify each chemical reaction as a combination, precipitation, single replacement, combus...

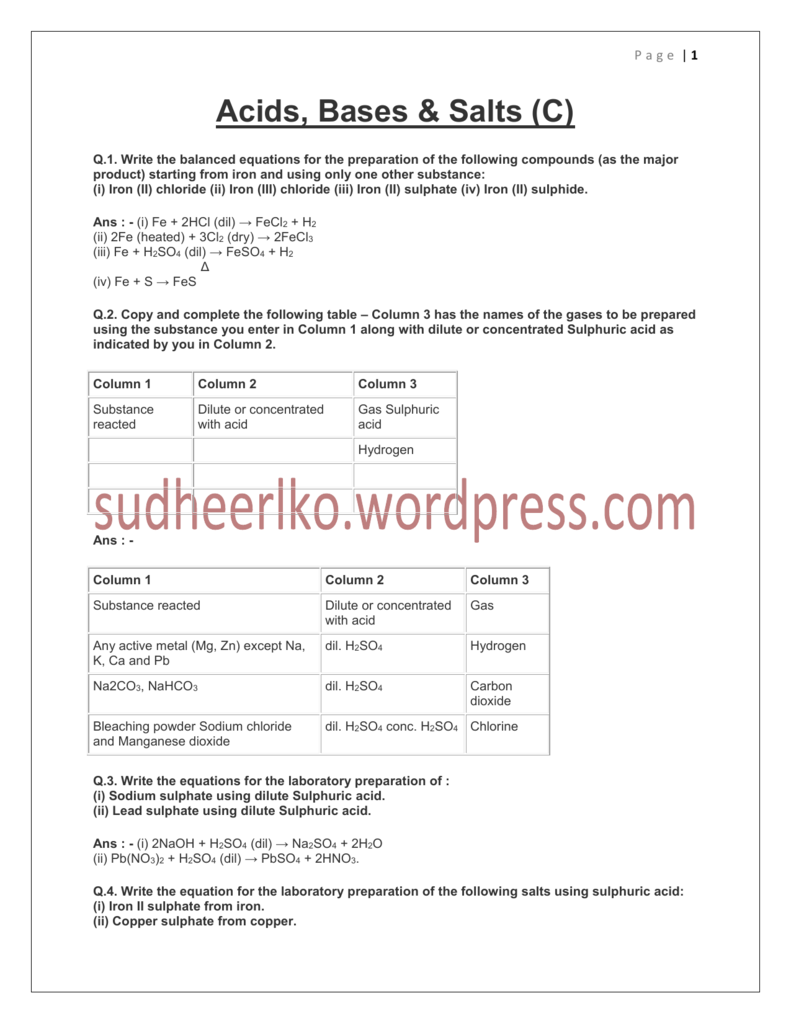
Write balanced chemical equations for the preparation of the following compounds (as the major product) starting from iron and using only one other substance: Iron (II) chloride

Match the following Cucl2 A) Direct cobination Fecl2 B) Neutralisation Fecl3 C) Precipitation Pbcl2 D) Acid+Metal Nacl E) Acid+Metal - Science - Acids Bases and Salts - 13894331 | Meritnation.com

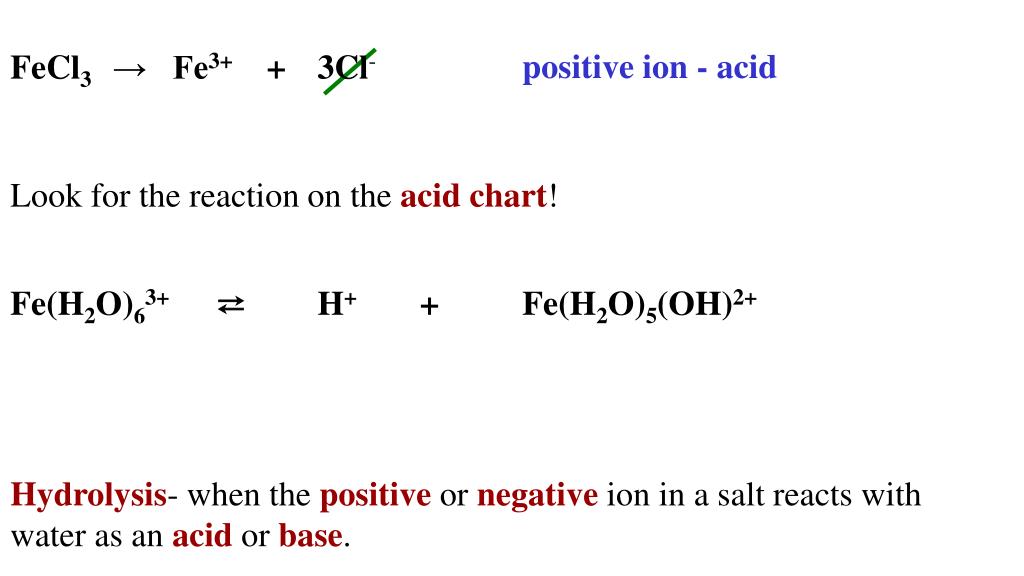
Acids and Bases Chapter 14 Acids and Bases. Acids and Bases Some Definitions Arrhenius Acid:Substance that, when dissolved in water, increases the concentration. - ppt download